Abstract
Background: Von Willebrand disease (VWD) is the single most common congenital bleeding disorder, affecting 1% of the population and characterized by deficient and/or defective von Willebrand factor (VWF). Among women with VWD, reproductive tract bleeding is common, with up to 80% developing postpartum hemorrhage (PPH), defined as 24-hr blood loss ≥500 cc after vaginal delivery, or ≥1,000 cc after cesarean delivery. Despite current guidelines suggesting 50 IU/kg plasma-derived VWF (pdVWF) at delivery if VWF:RCo is less than 0.50 IU/ml at the 8thmonth of gestation, PPH occurs even when that level has normalized, and, moreover, despite factor replacement, VWF levels are lower and blood loss greater at delivery than in controls. These data suggest VWF levels should be higher at delivery to prevent PPH. As blood volume increases 1.5-fold during pregnancy, and blood loss at delivery is 1.4-fold greater than controls, we have proposed a 1.5-fold higher dosing, ~80 IU/kg, to prevent PPH. Yet, experience with these agents at delivery is limited in women with VWD.
Methods: This was an observational study to compare postpartum blood loss in women with VWD, cared for at the Hemophilia Center of Western Pennsylvania, between February 1, 2017 and January 31, 2018, treated with recombinant von Willebrand factor (rVWF, Vonvendi®) or plasma-derived von Willebrand factor (pdVWF, Humate®). Medical records of all women with VWD undergoing delivery during the 12-month period were reviewed. The study was approved by the University of Pittsburgh IRB as an exempt study PRO18010198. Mean, median, and standard deviation or frequency (percentage) were determined for clinical variables including age, race, VWD type, bleeding score, bleeding history, body surface area, estimated blood loss, and comorbidity. Continuous variables were compared by student's t test, and discrete data were analyzed by chi-square or Fisher's exact test. A p-value of < 0.05 was considered statistically significant.
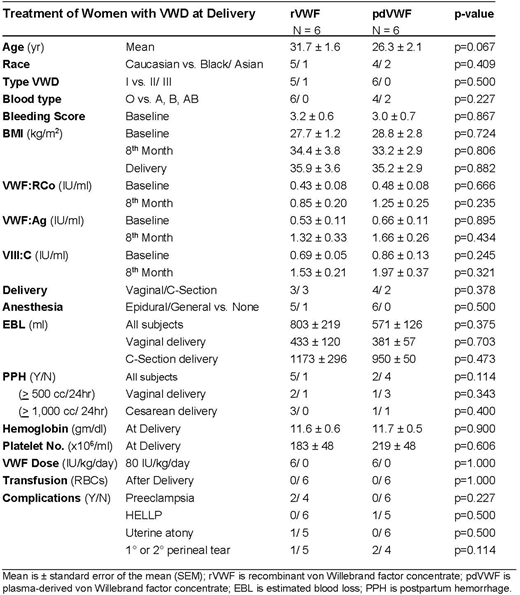
Results:A totalof 12 women with VWD, including 11 with type 1 and 1 with type 2B VWD, underwent delivery, 7 vaginal and 5 cesarean section, under coverage of clotting factor during the 12-month study period. These included 6 who received rVWF 80 IU/kg and 6 who received pdVWF 80 IU/kg at delivery and for up to 3 days postpartum. Treatment dose was based on pre-pregnancy weight and determined by insurance and patient choice. Demographic data were comparable between rVWF and pdVWF treatment groups, including age, 31.7 vs 26.3years (p=0.067); race, 5/6 vs 4/6 Caucasian (p=0.409); and blood type, 6/6 vs 4/6 type O (p=0.227). Bleeding history was similar between rVWF and pdVWF groups as were pre-pregnancy bleeding scores, 3.2 vs. 3.0(p=0.867). Co-morbidities were not different by treatment group, including anemia, p=.0227, diabetes, p=0.500, hypertension, p=0.500, obesity, p=1.00, and smoking, p=0.500. PPH occurred in 58.3% overall, with no difference in PPH by treatment group, overall, 5 of 6 (83.3%) vs 2 of 4 (50.0%) (p=0.114), and no difference by type delivery, vaginal (p=0.343) or cesarean delivery (p=0.400). Estimated blood loss by group in vaginal deliveries was 433vs 381 cc (p=0.703), and 1173 vs 950 cc (p=0.473) in cesarean. At delivery, groups had comparable hemoglobin, 11.6 vs 11.7, gm/dl, p=0.900, and platelet count, 183 vs 219 x106/ml (p=0.606), and none required transfusion. Length of stay was 3.8 vs 2.8 days (p=0.156). Comparing groups, VWF:RCo levels increased over 2.0-fold during pregnancy from baseline, 0.43 vs 0.48 (p=0.666), to 8thmonth, 0.85 vs 1.25 (p=0.235); VWF:Ag increased 2.5-fold from baseline, 0.53 vs 0.66 (p=0.895) to 8thmonth, 1.32 vs 1.66 (p=0.434); and VIII:C increased over 2.2-fold from baseline, 0.69 vs 0.86 (p=0.245); to 8thmonth, 1.53 vs 1.97 (p=0.321). BMI increased up to 1.3-fold from baseline, 27.7 vs 28.8 (p=0.882); to delivery, 35.9 vs 35.2 (p=0.882).
Conclusion:Postpartum blood loss was similar in rVWF and pdVWF treatment groups, whether vaginal or cesarean delivery. Dosing at 80 IU/kg appears safe, but over half developed PPH. Future trials are needed to evaluate blood-volume based VWF dosing to prevent PPH.
Ragni:Alnylam: Honoraria, Research Funding; Bayer: Consultancy, Provision of Drug for Investigator Initiated Trial; Biomarin: Consultancy, Honoraria, Research Funding; Bioverativ: Consultancy, Research Funding; CSL Behring: Research Funding; MOGAM: Consultancy; NovoNordisk: Research Funding; Opko Biologics: Research Funding; Sangamo: Research Funding; SPARK: Consultancy, Research Funding; Shire: Other: Provision of Drug for Investigator Initiated Trial; Institute for Clinical & Economic Review: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal